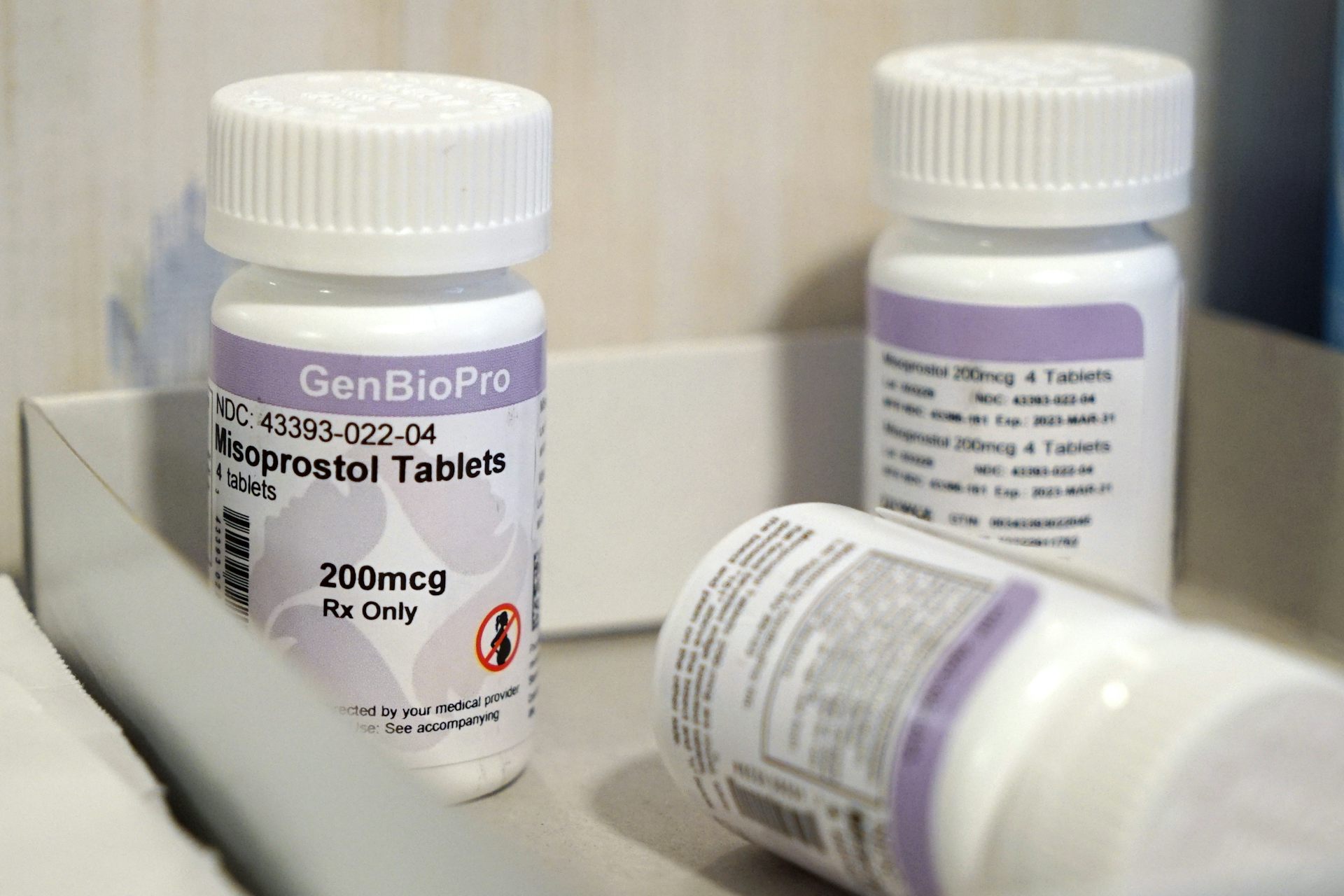
Louisiana’s Legislature approved a bill on May 23, 2024, that would reclassify two abortion pills, mifepristone and misoprostol, as “controlled, dangerous substances.” Both pills have a long history of safe and effective use in medication abortions as well as for treatment of miscarriages and other conditions. The bill, which is expected to be signed into law by the state’s governor, makes it illegal to possess either of the pills without a prescription. Surgical and medication abortions are already banned in Louisiana, with few exceptions.
The Conversation U.S. asked twin sisters Jamie Rowen, a legal scholar, and Tami Rowen, an obstetrician and gynecologist, to explain the new law’s implications – both for patients and providers.
What does Louisiana’s law mean for these abortion pills?
Mifepristone and misoprostol have long been classified as noncontrolled substances. Though a prescription is required in order to obtain them, there have been no criminal consequences for possessing these medications.
Louisiana’s new bill, once signed, would reclassify the pills as Schedule 4 drugs, which includes medications such as diazepam, more commonly known as valium, and tramadol, a commonly used opioid.
In the U.S., prescription medications are divided into two categories: not controlled and controlled. These are based on the drug’s likelihood for mental and physical addiction. Louisiana just moved the two medications from not controlled to controlled.
Access to medication abortion already requires a prescription everywhere in the U.S. So people seeking abortions will still need to get a prescription to obtain the pills under Louisiana’s new law, just as before.
What this law primarily does is make it a crime for people who are not seeking abortions to possess the pills. In Louisiana, it is against the law to provide a medical or surgical abortion absent the threat of a woman’s death or “substantial and irreversible bodily impairment.” Louisiana residents seeking medication abortion must get the pills from out of state. Under this new law, if someone without a valid prescription transports or stores mifepristone or misoprostol in Louisiana, they may face a penalty of between one and five years in prison and up to US$5,000 in fines.
Controlled substances are further categorized by schedules. Under federal law, schedule designations are determined by a drug’s potential for abuse, its risk to public health and a few other factors.
Under Louisiana state law, there are five schedules that follow similar categorizations as federal law. A Schedule 4 classification means that the drug or other substance has a low potential for abuse, has a currently accepted medical use, and that abuse of the drug or other substance may lead to limited physical dependence or psychological dependence.
By classifying mifepristone and misoprostol as a controlled Schedule 4 drug, the legislature is asserting without evidence that there are dependence and abuse risks associated with taking the medication.
What science, if any, did the Legislature rely on?
This is the first time that a state has classified abortion medication as a controlled substance, but it is not an unprecedented classification of abortion medication as a Schedule 4 drug.
Taiwan similarly classifies mifepristone this way.
The classification has no science behind it, however, as no studies suggest that the drug poses any risk of dependence or abuse. Mifepristone can be used for elective abortion, miscarriage management and even for labor induction, being more effective than traditional treatments with misoprostol alone.
In terms of safety, it is useful to compare the risks of mifepristone with another commonly used drug, Viagra. Though requiring a prescription, Viagra is not classified as a controlled substance. For mifepristone, there are rare reported cases of nonspecific fatal sepsis that could be related to women undergoing medical abortion in the U.S.
In contrast, Viagra was linked to 1,473 major adverse outcomes and 522 reported deaths in the U.S. during a 13-month period. The legislative history in Louisiana does not explain the science behind its classification scheme.
It should be noted that there is no risk of dependence from either misoprostol or mifepristone. Misoprostol was approved by the Food and Drug Administration for its ability to lessen the symptoms of gastric ulcers but is widely used throughout obstetrics and gynecology for inducing labor and stopping postpartum hemorrhage. Side effects for those using misoprostol for ulcers or gynecological care are rare, particularly compared with other Schedule 4 drugs.

How could the new law affect access for nonabortion procedures?
According to the new law, the criminal penalties of up to five years in prison can be imposed regardless of whether the pills are designated for a person electing abortion or trying to complete a miscarriage. In addition, the law does not distinguish between the possession of misoprostol for the treatment of gastric ulcers or gynecological care.
By punishing people who are transporting or storing the medications for others, the law may create new barriers to access for a variety of people, even if it doesn’t criminalize pregnant people who consume the pills.
What are the implications of the laws for other states?
The Supreme Court has interpreted the Constitution as allowing both the federal government and the states to regulate controlled substances. This means there can be variations in access to medications state to state.
However, states typically follow federal scheduling guidelines and practices when making their own drug classifications. A well-known but rare exception to this common practice is cannabis, which many states have decriminalized or legalized contrary to federal law.
The immediate impacts will be on clinical studies on the two drugs in other states that have patients in Louisiana. Clinical trials with medications that are not controlled substances may be conducted in any state with little or no added state regulation. Clinical trials of controlled substances have additional regulatory requirements that can add costs and delay development.
The most likely long-term outcome is that other states may make similar legislative attempts to curb access to medication abortion, particularly in states that have unsuccessfully tried to stop access to medical abortion in other ways.
![]()
The authors do not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and have disclosed no relevant affiliations beyond their academic appointment.